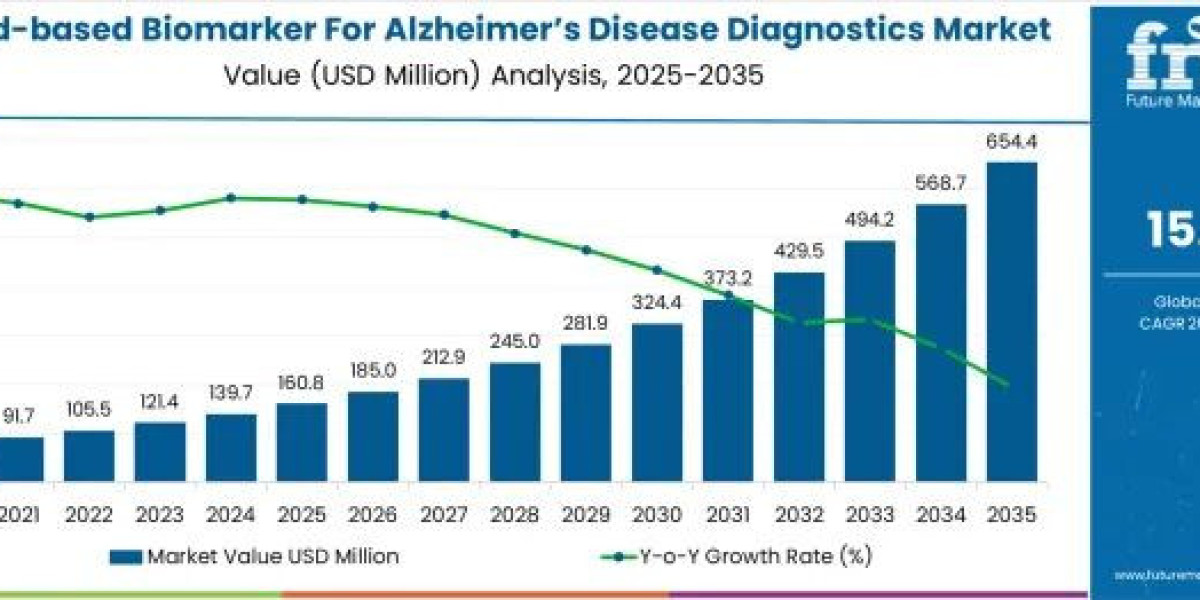
In a landscape where the global burden of Alzheimer's disease continues to escalate, the emergence of blood-based biomarkers is heralding a new era of accessible, early detection. The blood-based biomarker for Alzheimer's disease diagnostics market is on track for remarkable expansion, forecasted to grow from USD 160.8 million in 2025 to an impressive USD 654.4 million by 2035. This trajectory reflects a robust compound annual growth rate (CAGR) of 15.1%, underscoring the sector's potential to revolutionize preventive neurology and precision medicine.
As populations age worldwide, the demand for non-invasive diagnostic tools has never been more urgent. Traditional methods, such as cerebrospinal fluid analysis and costly brain imaging, have long posed barriers to widespread screening. Blood-based biomarkers offer a game-changing alternative, enabling early identification of Alzheimer's pathology through simple blood samples. This shift not only enhances patient accessibility but also aligns with the growing emphasis on proactive healthcare strategies. Industry leaders are increasingly recognizing the value in integrating these innovations into routine clinical workflows, driving adoption across diverse settings from primary care to specialized memory clinics.
Read Full Report-https://www.futuremarketinsights.com/reports/blood-based-biomarker-for-alzheimers-disease-diagnostics-market
The market's momentum is fueled by several key drivers. Foremost is the rising prevalence of Alzheimer's, which affects millions globally and places immense strain on healthcare systems. Technological breakthroughs in proteomics and immunoassays are accelerating biomarker development, allowing for ultra-sensitive detection of disease indicators like tau proteins and amyloid aggregates. Moreover, the integration of artificial intelligence (AI) and machine learning is enhancing diagnostic accuracy, enabling predictive analytics for cognitive decline. Governments and healthcare organizations are prioritizing dementia care through increased funding for neurological research, while regulatory pathways are evolving to support faster validation and approval of these tools.
However, the path forward is not without challenges. Regulatory complexities in biomarker validation remain a hurdle, requiring rigorous clinical trials to ensure reliability and standardization. Reimbursement uncertainties in various regions could slow adoption, particularly in resource-constrained settings. Despite these restraints, opportunities abound. The expansion of large-scale screening programs and the push toward point-of-care testing platforms promise to democratize access, especially in emerging markets. Precision medicine initiatives are also opening doors for combination biomarker panels that provide comprehensive insights into disease progression.
Delving into market segmentation reveals strategic insights for decision-makers. By biomarker type, tau-related markers dominate with a 37% share in 2025, prized for their strong correlation with neurodegeneration and utility in staging and prognosis. Amyloid-related and neurodegeneration markers follow, contributing to a multifaceted approach in diagnostics. In terms of technology, immunoassays lead with approximately 54% market share, thanks to their established automation, high throughput, and reproducibility. Mass spectrometry-based assays and next-generation platforms are gaining traction for their precision in complex analyses.
End-user dynamics highlight the central role of clinical laboratories and hospital labs, capturing 53% of the market in 2025. These facilities benefit from integrated infrastructure and alignment with centralized testing models. Pharma and biotech companies, along with academic and research institutes, are pivotal in driving innovation through R&D collaborations. This segmentation underscores the need for tailored strategies: clinical labs focus on scalability, while research entities prioritize cutting-edge validation.
Regionally, the market narrative is one of diverse growth patterns, with Asia Pacific emerging as a powerhouse. China leads with a staggering 20.3% CAGR from 2025 to 2035, propelled by its rapidly aging population, expanding hospital networks, and local development of immunoassays and proteomic panels. India follows at 18.8%, supported by public health initiatives and cost-optimized assays in specialized centers. In Europe, Germany (17.3% CAGR) stands out due to its favorable reimbursement environment and EU compliance standards, while France (15.8%) and the UK (14.3%) leverage national dementia strategies and NHS pilots. North America, particularly the USA at 12.8% CAGR, benefits from federal funding, commercial lab networks, and consortia establishing benchmarks. Latin America, exemplified by Brazil's 11.3% growth, is advancing through public hospital pilots and university partnerships. These regional variances offer industry leaders opportunities to customize go-to-market strategies, from regulatory navigation in Europe to affordability-focused innovations in Asia.
The competitive landscape is concentrated yet dynamic, with the top five players commanding 61-66% of the global share. F. Hoffmann-La Roche Ltd leads with 22%, leveraging its expertise in neurodegenerative research and validated immunoassays. Quanterix excels with proprietary SIMOA platforms for ultra-sensitive detection, while Fujirebio advances mass-spectrometry assays. C2N Diagnostics offers CLIA-validated multi-marker panels, and Labcorp and Quest Diagnostics dominate through expansive diagnostic networks. Other notables include Siemens Healthineers for high-throughput analyzers, Abbott for assay kits, Lilly USA LLC in biotech, and ALZpath for next-generation phosphorylated tau assays. Strategies emphasize clinical sensitivity, specificity, and workflow integration, with recent developments including AI-enhanced panels, regulatory approvals, and licensing deals.
Analysts view this market as transitioning toward scalable, early-stage diagnostics that could significantly reduce the societal impact of Alzheimer's. The focus on real-world evidence, cost-effectiveness, and AI-driven risk assessment positions blood-based biomarkers as essential tools in the fight against dementia.
Subscribe for Year-Round Insights → Stay ahead with quarterly and annual data updates: https://www.futuremarketinsights.com/reports/brochure/rep-gb-26329
Request for Discount: https://www.futuremarketinsights.com/reports/sample/rep-gb-26329
As we stand on the cusp of this transformation, stakeholders must act decisively. Investments in R&D, strategic partnerships, and policy advocacy will be key to unlocking the full potential of these innovations. For healthcare providers, adopting blood-based testing means earlier interventions and better patient outcomes. For pharma leaders, it opens avenues for targeted therapies. And for policymakers, it represents a step toward equitable dementia care worldwide.
This narrative not only highlights the market's exponential growth but also humanizes the impact: behind the numbers are millions of lives that could be improved through timely diagnosis. As the sector evolves, blood-based biomarkers are poised to become the cornerstone of Alzheimer's management, fostering a future where early detection is the norm, not the exception.
Explore More Related Studies Published by FMI Research:
Semen Warmer Market- https://www.futuremarketinsights.com/reports/semen-warmer-market
Avian Influenza Antigen Testing Market- https://www.futuremarketinsights.com/reports/avian-influenza-antigen-testing-market
Dialysis Water Filters Market- https://www.futuremarketinsights.com/reports/dialysis-water-filters-market
Newborn Name Tag Market- https://www.futuremarketinsights.com/reports/newborn-name-tag-market
About Future Market Insights (FMI)
Future Market Insights, Inc. (FMI) is an ESOMAR-certified, ISO 9001:2015 market research and consulting organization, trusted by Fortune 500 clients and global enterprises. With operations in the U.S., UK, India, and Dubai, FMI provides data-backed insights and strategic intelligence across 30+ industries and 1200 markets worldwide.
Why FMI: Decisions that Change Outcomes- https://www.futuremarketinsights.com/why-fmi
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-347-918-3531
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
Have a specific Requirements and Need Assistant on Report Pricing or Limited Budget please contact us - sales@futuremarketinsights.com